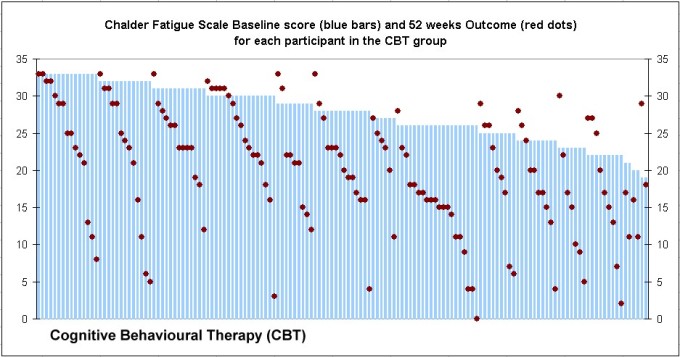
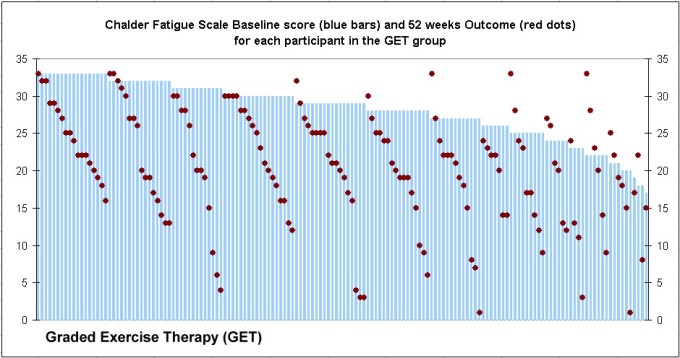
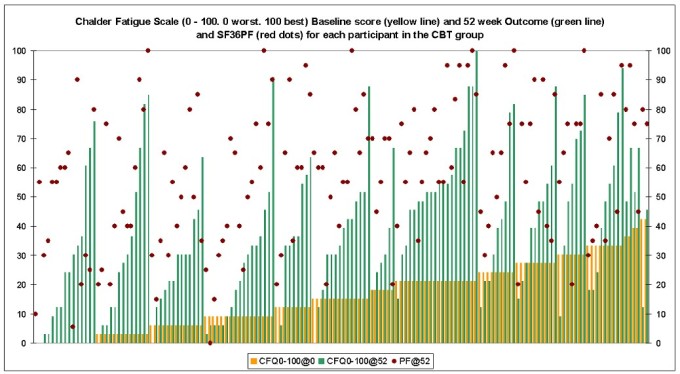
“Clinical and cost-effectiveness of the Lightning Process in addition to specialist medical care for paediatric chronic fatigue syndrome: randomised controlled trial”
(Crawley EM, Gaunt DM, Garfield K, et al. Clinical and cost-effectiveness of the Lightning Process in addition to specialist medical care for paediatric chronic fatigue syndrome: randomised controlled trial [published correction appears in Arch Dis Child. 2019 Jul 11;:] [published correction appears in Arch Dis Child. 2019 Jul 11;:]. Arch Dis Child. 2018;103(2):155–164. doi:10.1136/archdischild-2017-313375)
The authors of the LP trial state that with the SF-36 Physical Function subscale (SF-36PF) “Higher score=fewer symptoms, better function.” This is incorrect.
The SF-36PF provides no information about the number of symptoms that a participant has. A participant with one symptom, e.g., severe chronic pain, could have an SF-36PF score of zero, equivalent to being ‘limited a lot’ for all 10 items in the questionnaire. Whereas a person with multiple symptoms could score 100, showing that they are not limited at all in carrying out any of the activities.
As this misrepresentation of the properties of the SF-36PF is obviously made-up, it is reasonable to question whether the authors intended to exaggerate the importance of their primary outcome measure, which is further suggested by the chart which they published for the measure. Figure 1 shows how the SF-36PF primary outcome measure data was represented in the LP paper, and figure 2 shows the same data in a full scale (0 to 100) chart, which does not exaggerate the small difference between the LP group and the Specialist Medical Care (SMC) control group.
Figure 1. SF-36PF data as illustrated in the LP trial

Figure 2. SF-36PF data from Figure 1 in a full scale chart

There was a 14 point difference between the mean SF-36PF outcomes of the LP group and control group which is potentially interesting. The LP group had an average outcome rating of 86, yet the mean school attendance for this group was only 4 days out of 5. While this was one day more than the control group, missing 20% of schooling one year after being given the LP is very poor. It is also notable that an average SF-36PF rating of 86 is disturbingly low for a group of formerly healthy young people (see Table 1 for an example of the questionnaire which produces a rating of 85). For example, an SF-36PF rating of 85 could represent:
- “limited a lot” in “Vigorous activities, such as running, lifting heavy objects, participating in strenuous sports”,
- AND “limited a little” in “Moderate activities, such as moving a table, pushing a vacuum cleaner, bowling, or playing golf”.
- And an SF-36PF rating of 80, could correspond with both of the above PLUS: “limited a little” in “Walking more than one mile”.
Although these are unexpected limitations for young people, a rating of 80 or 85 does not allow for any limitations at all with any of the other activities listed, which includes being able to walk several blocks (approximately half a mile or 770 metres), climbing several flights of stairs and lifting and carrying groceries.
Which raises the question: if the average LP group participant was “not limited at all” in any of the SF-36PF lighter activities – why was their average school attendance only 4 days out of 5?
Table 1. SF-36 Physical Function subscale. Example illustrating a score of 85.

A possible explanation for this contradiction, would be that the mental-conditioning that the young people underwent, programmed them to say and to believe, that they are healthier and more able-bodied than they actually are. It might not be difficult to produce this effect because to a degree, it seems to occur naturally. For example, Dr David Bell wrote an article on the ‘Prognosis of ME/CFS’, which discussed his and his colleague’s studies of patients who became ill in their youth. On the 25 year follow-up of patients, Bell observed: “All studies employed the Rand-36 (SF-36), a questionnaire is [sic] common use. The first question of this instrument is ’how would you rate your health?’ Many of the patients rated their health as ‘good’, while the rest of the questionnaire demonstrated how poorly they were actually functioning.” Dr Bell concluded his essay: “Forty year old adults who had an acute onset during their teenage years have the activity of seventy year old adults. Until there is a reliable biomarker for the severity of ME/CFS, we will be unable to accurately define the prognosis.”1
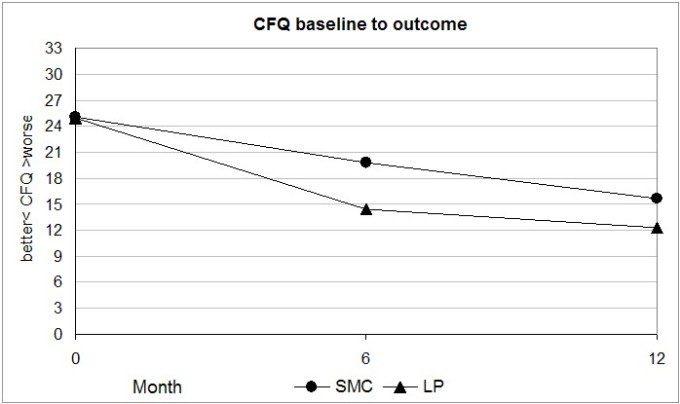
The LP group mean Chalder Fatigue Questionnaire (CFQ) rating at outcome was 12.3 as shown in Figure 2. This shows that on average, only one or two fatigue items were a problem ‘more than usual’. Table 2 illustrates a CFQ producing a rating of 13.
The average LP participant reported only one or two (out of 11) CFQ factors as being, ‘worse than usual’, and indicated no limitations at all in carrying out light physical activities with the SF-36PF, yet the group were STILL missing 20% of school attendance.
Table 2. The Chalder Fatigue Questionnaire (CFQ). Example showing a score of 13

Figure 2. LP trial Chalder Fatigue Questionnaire data, baseline to outcome.
A possible explanation for these contradictory outcomes, is that the LP made no actual difference to the participant’s fatigue and physical functioning – which as a group average was improving anyway, as evidenced by improvements in the control group. But what the LP might have done, is influence the way that the participants perceived and subjectively rated their physical limitations and fatigue. The evidence suggests that this occurred, because youngsters reporting an average SF-36PF rating of 85 and CFQ of 12 should not be missing any more school than fully fit children.
An alternative or additional explanation, could be that a small proportion of 10% to 20% of participants were suffering from depression or an anxiety disorder caused by the stress of their illness. Stress is a common consequence of chronic illness.2 So it is possible that a small proportion of participants got some benefit if the LP happened to help with these issues. This could easily result in the data of a few participants skewing the results and creating the appearance of a treatment-effect, despite the LP having no effect whatsoever on the actual causes of CFS. Had the researchers reported the trial in more detail and included some defined thresholds, even if only for post-hoc analysis, the data might have shown whether this actually occurred.
In my opinion, the LP rather cleverly exploits two phenomena – either of which could confound any serious evaluation of the effectiveness of a therapy. Firstly, the LP exploits ‘client deference’,3 a phenomenon which can prevent clients from raising concerns that they might have about their therapy or therapist, by depriving them of ‘agency’ or autonomy. It is a phenomenon which can subjugate the client’s will to that of a therapist. Secondly, the LP puts the entire responsibility for the success or failure of the therapy on to the client, thereby exploiting a ‘self-serving bias’ when the client comes to rate their progress, because the client is effectively rating their own effort and commitment to getting better.4 The simple and scientific way to prevent these phenomena from confounding the research would have been to include objective measures of physical function such as the Six Minute Walk Test.5
Finally, I cannot help wondering if some of the researchers got hypnotised during the trial. The published findings are selective and exaggerated and the interpretations suggest a lack of critical and objective analysis. The paper states that: “there was good evidence that SMC+LP was more cost-effective than SMC alone”. This round-about claim that the LP is an effective treatment for CFS is not supported even by the published data. ‘Good evidence’ that the LP was effective would be participants attending ALL their schooling and being able to do the same sports that healthy kids enjoy. These objective outcomes would show that the LP accurately and effectively addressed the underlying cause(s) of participants’ CFS. But the evidence shows that the LP got nowhere near these results. Instead, the evidence shows that the average LP participant still had substantial physical limitations, they were often absent from school and were only marginally and subjectively better than an average control participant. Furthermore, the evidence suggests that some of the LP participants acquired a distorted and over-optimistic perception of their health and physical abilities which influenced how they rated subjective measures.
Peter Kemp
September 2019
REFERENCES
- Bell D. Prognosis of ME/CFS. The Open Medicine Foundation. Aug 1 2016. Online. Available at: https://www.omf.ngo/2016/08/01/prognosis-of-mecfs/. Accessed February 25 2017.
- The Burden of Stress in America. Robert Wood Johnson Foundation and Harvard School of Public Health. July 2014. Online. Available at: https://cdn1.sph.harvard.edu/wp-content/uploads/sites/21/2014/07/Burden-of-Stress-Report-July-7-2014.pdf. Accessed Sept 16, 2019.
- Rennie, D. Clients’ Deference in Psychotherapy. Journal of Counseling Psychology. 1994, Vol. 41, No. 4, 427-437.
- Self-Serving Bias. Wikipedia. Online. Available at: https://en.wikipedia.org/wiki/Self-serving_bias. Accessed Sept 16, 2019.
- Ulrich S, Hildenbrand FF, Treder U, et al. Reference values for the 6-minute walk test in healthy children and adolescents in Switzerland. BMC Pulm Med. 2013;13:49. Published 2013 Aug 5. doi:10.1186/1471-2466-13-49. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3750631/