Analysis and opinion by Peter Kemp MA
September 2016
[Emphases throughout are added]
This article follows-on from my Blog:
The PACE Trial ‘Normal Range’ – an Untenable Construct
https://wordpress.com/post/peterkempblog.wordpress.com/10
which analysed the PACE Trial authors’ justifications for discarding the Protocol Primary Outcome Measures and use of ‘Normal Range’ as a measure of efficacy for treatments.
As a reminder, here is how ‘Normal Range’ compares with the Primary Outcome Measures which the PACE Trial authors’ discarded. The chart is adapted directly from one published in The Lancet, with the addition of orange lines showing outcome thresholds. The blue line shows 3 times the difference between SSMC (Control Group) and GET/CBT. The Protocol anticipated that the difference between SSMC and GET/CBT would be 2 to 3 times, which would indicate a clinical improvement over and above the control group.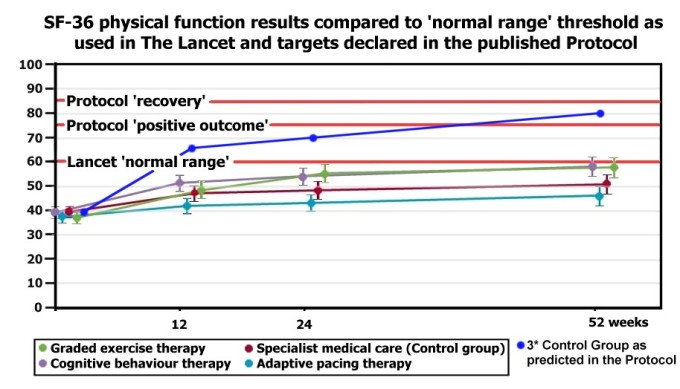 And below is a table illustrating various interpretations of the ‘Normal Range’ SF36PF (Short Form 36 Physical Function subscale) threshold, showing that it is clinically meaningless, even to those that designed and employed it.
And below is a table illustrating various interpretations of the ‘Normal Range’ SF36PF (Short Form 36 Physical Function subscale) threshold, showing that it is clinically meaningless, even to those that designed and employed it.
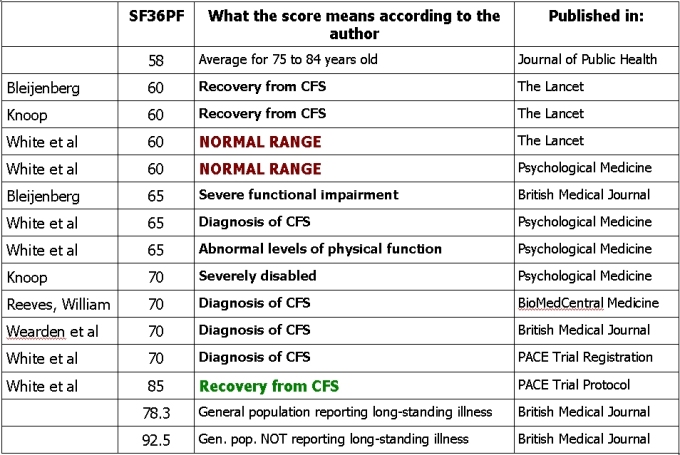
(White was the PACE Trial chief investigator, Bleijenberg and Knoop published a comment on the PACE Trial accompanying the PACE report in the Lancet, Wearden was the FINE Trial chief investigator, Reeves was the head of the CFS department at the CDC)
The Rights of Participants
Misleading professionals and the public is not the only effect of misrepresenting research. In 2006 Sir Iain Chalmers, one of the founders of the Cochrane Collaboration, commented: “I published an article with the deliberately provocative title ‘Underreporting research is scientific misconduct’. I suggested that failing to report well-conducted clinical trials was not only scientific misconduct, but also unethical; it broke an implicit contract with the patients who had participated in clinical trials.”[1]
Participants are sought for clinical trials because they suffer with the illness under investigation. They are entitled to expect that medical and scientific knowledge will advance by their participation through the accumulation and dissemination of accurate information. Exploiting the trust and generosity of participants by misrepresenting their data is unethical.
The MRC [Medical Research Council] Guidelines for Good Clinical Practice in Clinical Trials states:
“2. THE PRINCIPLES OF GOOD CLINICAL PRACTICE FOR MRC-FUNDED TRIALS
2.4 The rights, safety and well-being of the trial participants are the most important consideration and should prevail over interests of science and society.”
When the PACE Trial published its first Participant Newsletter in June 2006[2], it took the dubious step of publishing some participants’ comments. These included: “I really think it is good to be part of something that will make a difference to so many people”, and: “We need this research to know the best treatments”.
These comments illustrate the type of participant motivation that researchers rely upon for recruitment – supporting scientific advancement and helping others. They indicate that participants should be considered to have social and moral values that could be offended by being involved in any form of deception.
The Declaration of Helsinki states: “11. It is the duty of physicians who participate in medical research to protect the life, health, dignity, integrity, right to self-determination, privacy, and confidentiality of personal information of research subjects.”
The MRC Guidelines for Good Research Practice state: “All research supported by the MRC must respect and maintain the dignity, rights, safety and wellbeing of all involved, or who could be affected by it.”
In March 2007 the PACE Trial Protocol was published as an ‘open access’ document in BioMedCentral Neurology and was freely available to over 80% of participants before or during their participation. The Protocol should therefore be considered to form part of the Participant Information and the contract of Informed Consent. Any deviation from the Protocol that could affect participants’ motivation, integrity or values without their agreement nullifies their Informed Consent.
No Research Ethics Committee, Trial Steering Committee or Trial Management Group has the right to supersede an individual’s right to provide or withhold Informed Consent.
This is because ‘Informed Consent’ requires by definition, that participants are in possession of all relevant information, including foreseeable risks to themselves and others, necessary to make an informed decision. Therefore, any PACE Trial participant who did not receive and understand a full explanation of the implications of the changes to the Protocol, had not given Informed Consent. They would instead be subjects of Human Medical Experimentation without consent.
When major alterations are made to a Research Protocol, “which could affect the personal integrity and/or welfare of trial participants” (MRC 5.2.1) they must be consulted and provided with an explicit opportunity to withdraw or provide new consent. If this is not done, then it is reasonable to suggest that the effects and repercussions of alterations to the Protocol have been withheld from participants.
The data for PACE Trial participants whose SF36PF score was 60 and whose physical limitations were far worse than those required for a diagnosis of M.E. or CFS, was suddenly transformed from ‘Severe functional impairment’ and ‘Abnormal levels of physical function’, into ‘normal range’. Did participants know and understand that this was being done to their data or what the implications could be for themselves or others?
A participant whose SF36PF score was 60 would evidently be severely restricted in all activities. How would they feel about scientific journals and other media claiming that they were ‘normal range’ or ‘recovered’? How might it impact their medical support or entitlement to state benefits? How might it affect their relationships with family and friends? What impact could it have on their trust of medical professionals and researchers if their illness and disability are so misrepresented?
The General Medical Council Guidelines for Good Practice in Research state:
“Sharing information
“4 You must give people the information they want or need in order to decide whether to take part in research. How much information you share with them will depend on their individual circumstances. You must not make assumptions about the information a person might want or need, or their knowledge and understanding of the proposed research project.”
The PACE Trial Participant Information Sheet states:
“What is your study for?
“There are different treatments for CFS/ME, and we want to know which are the most helpful. To find this out, we are asking people like you who suffer from CFS/ME to join our study – which is a randomised controlled trial.
“We hope our study will tell us about the benefits and possible drawbacks of each of these treatments. We also hope to learn why successful treatments work and whether different people need different treatments.”
The PACE Trial Participant Information Sheet informed potential participants that the research was to find out whether or not the treatments are beneficial. The evidence shows that changing the Primary Outcome Measures actually obstructed the dissemination of useful, accurate information about the treatments, but instead facilitated widespread misreporting of the treatments and misrepresentation of participant data.
The PACE Trial Protocol states:
“Methods/Design Aims
The main aim of this trial is to provide high quality evidence to inform choices made by patients, patient organisations, health services and health professionals about the relative benefits, cost-effectiveness, and cost-utility, as well as adverse effects, of the most widely advocated treatments for CFS/ME.”
When researchers misrepresent or allow the misrepresentation of data from trial participants, it exploits the trust and generosity of the volunteers and involves them, unwittingly and against their will, in misleading other patients, medical professionals and the public. PACE Trial volunteers got no ‘meaningful clinical difference’ to their health from their participation. Just 15% may have benefited slightly, probably due to the individual support and attention they received. 85% got no treatment effect at all. During the period of the trial, 52 experienced ‘serious deterioration’ and 42 had ‘serious adverse events’. All were called upon to donate substantial amounts of time and effort. Yet a very high proportion sustained participation for the full 52 weeks, completing numerous questionnaires and keeping regular appointments. These volunteers deserve better than to be misrepresented in the Lancet and the worldwide media.
False and misleading reports about PACE Trial participants
The MRC Guidelines for Good Research Practice state:[3] “G.6 The final results of MRC-funded research must be collated, summarised and subjected to quality assurance and, where appropriate, peer review. A conclusion should be drawn and the outcome confirmed by the research team. The MRC encourages the publication of all research findings, including findings that do not support the initial hypotheses to allow others to benefit from the work and to avoid unnecessary repetition.”
The following are examples of misleading reporting of the PACE Trial by National and International media and authorities:
The Daily Mail: Got ME? Fatigued patients who go out and exercise have best hope of recovery, finds study
The Independent: Got ME? Just get out and exercise, say scientists
The Guardian: Study finds therapy and exercise best for ME
The Telegraph: Exercise and therapy can help ME sufferers, study claims
The Daily Express: TRIAL OFFERS HOPE FOR ME SUFFERERS
The Daily Record: Exercise and therapy can reverse effects of ME
The Medical Research Council
http://www.mrc.ac.uk/Newspublications/News/MRC007706
UK’s largest CFS/ME trial confirms safe and effective treatments for patients.
- Two effective treatments benefit up to 60 per cent of patients with Chronic Fatigue Syndrome or Myalgic Encephalomyelitis (CFS/ME), according to a collaborative trial funded by the Medical Research Council (MRC) and UK government departments.
Kings College London
http://www.kcl.ac.uk/iop/news/records/2011/feb2011/SafetreatmentsforCFSME.aspx
- Two effective treatments benefit up to 60 per cent of patients with Chronic Fatigue Syndrome or Myalgic Encephalomyelitis (CFS/ME)
- Professor Trudie Chalder from King’s College London and a co-author, said: ‘We have found that both CBT and GET can safely help a significant number of patients…”
Queen Mary University of London: http://www.qmul.ac.uk/media/news/items/smd/44140.html
- Two effective treatments benefit up to 60 per cent of patients with Chronic Fatigue Syndrome or Myalgic Encephalomyelitis
CBC News. Radio Canada
http://www.cbc.ca/news/health/story/2011/02/18/chronic-fatigue-exercise-behaviour-therapy.html
Chronic fatigue may be reversed with exercise
- Taking it easy is not the best treatment for chronic fatigue syndrome, rather exercise and behaviour therapy are, a large study finds.
- Knoop said the behavioural and exercise therapies may have worked by convincing patients they can recover, leading to an actual improvement.
- Still, the treatments only helped about 60 per cent of patients and researchers were unsure how long the results lasted.
- “Even with the best therapies we have, four out of 10 people don’t improve,” said Peter White, a professor of psychological medicine at the Queen Mary University of London, who led the study.
Primary Care Reports: 18 February 2011
http://www.primarycarereports.co.uk/uk.html
- UK’s largest CFS/ME trial confirms safe and effective treatments for patients
- Two effective treatments benefit up to 60 per cent of patients with Chronic Fatigue Syndrome or Myalgic Encephalomyelitis (CFS/ME), according to a collaborative trial funded by the Medical Research Council (MRC) and UK government departments.
NBC News. USA.: http://www.msnbc.msn.com/id/41651311/ns/health-health_care/#.UNHy5uTtRXM
- Study suggests exercise, behavior therapy helps more than just taking it easy
- The findings also suggests the crippling condition can sometimes be reversed.
- Still, the treatments only helped about 60 percent of patients and researchers were unsure how long the results lasted.
- “Even with the best therapies we have, four out of 10 people don’t improve,” said Peter White, a professor of psychological medicine at the Queen Mary University of London, who led the study.
Clinical Psychiatry News: http://tinyurl.com/grdmf3b
“The take-home message is that we now have robust evidence of the effectiveness, and importantly for patients, the safety of CBT and GET, as long as they are given by properly trained people,” said Dr. Michael Sharp[sic], a study coauthor who is a professor of psychological medicine and director of psychological medicine research at the University of Edinburgh, Scotland
Therapy Today: (journal of the British Association of Counsellors and Psychotherapists) http://www.therapytoday.net/article/show/2317/
Hope for ME sufferers as study results support effective therapy
FOX News: http://tinyurl.com/jskwdl6
Helping chronic fatigue syndrome patients to push their limits and try to overcome the condition produces a better rate of recovery…up to 60 percent of patients improved… The results showed that CBT and GET benefited up to 60 percent of patients, and around 30 percent of patients in each of these treatment groups said their energy levels and ability to function and returned to near normal levels.
The Universal Ethical Code for Scientists (as referred to in MRC Guidelines) states:[4] “Do not knowingly mislead, or allow others to be misled, about scientific matters. Present and review scientific evidence, theory or interpretation honestly and accurately.”
The MRC Guidelines for Good Research Practice state: “The findings of MRC-funded research must be made available to the research community and the public, in a timely manner. A complete, balanced and accurate account of scientific evidence must be presented to support the appropriate and effective use of this knowledge.”
The use of repeated phrases and statistics suggest that the media reported information that they were given directly or indirectly by the PACE Trial investigators via the Science Media Centre (ScMC) press conference or ScMC ‘expert reaction’.
Here are some quotes from the media coverage:
- Helping chronic fatigue syndrome patients to push their limits and try to overcome the condition produces a better rate of recovery…
- With cognitive behavioural therapy, 30% of patients returned to normal levels of fatigue and physical function.
- The results showed that CBT and GET benefited up to 60 percent of patients, and around 30 percent of patients in each of these treatment groups said their energy levels and ability to function and returned to near normal levels.
- ME sufferers have been offered new hope following a landmark study which suggests the condition can be reversed with counselling and exercise.
- The ground-breaking study is the most comprehensive to date and challenges the widely accepted belief that the illness cannot be cured.
- Researchers found six in 10 patients reported significant improvements after undergoing either cognitive behavioural therapy (CBT) – a type of counselling…
- With cognitive behavioural therapy, 30% of patients returned to normal levels of fatigue and physical function.
- Overall, 60 per cent of patients who received CBT or GET made progress and 30 per cent recovered sufficiently to resume normal lives.
- Although the numbers who recovered were small, Trudie Chalder, professor of cognitive behavioural psychotherapy at King’s College, London, said that “twice as many people on graded exercise therapy and cognitive behaviour therapy got back to normal” compared with those in the other two treatment groups.
- Six in 10 patients reported significant improvements, with half reporting a return to normal energy levels.
The repeated words, phrases and statistics in these UK mainstream media articles include:
- “6 in 10” or “60 percent” (7)
- “half of these” or “30 percent” (8)
- “can be reversed” (7)
- “challenges the … belief that the illness cannot be cured” (3).
- “got back to normal” or “near normal” or “normal levels” or “normal lives” or “return to [or: to near] normal energy levels” (9)
- “cure” or “cured” or “recover” or “recovered” (9)
The claims of ‘recovery’, ‘return to normal’ and figures of 60% and 30% attributed to GET or CBT are all misleading. These false claims appeared in the media around the world. The repetition of figures and terms suggests that they originated from a common source.
The MRC Scientific misconduct policy and procedure states:[5]
“2. Definition of Scientific Misconduct
2.1 Scientific Misconduct means fabrication, falsification, plagiarism or deception in proposing, carrying out or reporting results of research and deliberate, dangerous or negligent deviations from accepted practice in carrying out research. It includes failure to follow established protocols if this failure results in unreasonable risk or harm to humans…”
The MRC Good research practice states:[6] “G.5 When reporting research findings in publications, presenting at scientific meetings and engaging in debates in the media or in public, any relevant interests must be declared. This is to help others understand the factors that may have influenced the research team and would include any interests that might be considered by others, including the public, to be a conflict.”
Conclusion
The media reports about the PACE Trial are false and misleading. There is no evidence that anyone involved in the PACE Trial or their institutions have taken action to correct this wholesale misrepresentation of their research.
It appears that PACE Trial participants have been exploited by being involved without their knowledge or consent in misleading the media, medical professions and the public. The PACE Trial publicity has concealed from patients, professionals and the public, the ‘clinically important’ outcome of the research which ‘Positive Outcome’ and ‘Recovery’ were specifically designed to provide. Given the evidence that ‘Normal Range’ is clinically meaningless and that its contrivance has made the PACE Trial an example of ‘How Not to do Research’, the glaring contradictions of the PACE authors’ explanations, and the lottery-winning coincidence that ‘Normal Range’ just happened to fall in the exact spot to create the appearance of a treatment effect – the whole project looks like a huge waste of time and money. But worst of all, it appears to have betrayed the trust of participants.
Peter Kemp MA
Sept 2016
References
Some reference hyperlinks may be out of date but should be easily recoverable by searching Google with quoted phrases inside speech marks.
[1] Iain Chalmers. JRSM 2006; 99:337–341. doi:10.1258/jrsm.99.7.337.
[2] Online: http://www.pacetrial.org/docs/participantsnewsletter1.pdf
[3]Online:http://www.mrc.ac.uk/Ourresearch/Ethicsresearchguidance/Researchpractice/principles_guidelines/index.htm
[4] Online: http://www.berr.gov.uk/files/file41318.pdf
[5] Online: http://www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC005820
[6]Online:http://www.mrc.ac.uk/Ourresearch/Ethicsresearchguidance/Researchpractice/principles_guidelines/index.htm
This was brilliant work and really exposes the lies of the PACE trial authors and how powerful the media is when it runs with those lies. Thank you.
LikeLiked by 1 person
Yes I believe they were exploited, nothing about this study was truely about helping those who have ME/CFS but to put out biased info when the study didnt have the results those researchers were after.
This study was a huge problem for this patient group world wise as doctors all over the world have acted on it and governments have made policies based on this info. So many who have ME/CFS have been harmed.
In my own case here in Australia this could of indirectly lead to me getting into a car accident where I hit another car. Someone could of been killed from this.
I got knocked back on getting the disability pension I needed when I was very severely ill due to not having had treatment. As I was in fact getting treatment at the time by doctor prescribing things but it wasnt GET or CBT, we can only assume that knock back from the centrelink assessor for lack of treatment could only been him refering to me not having the recommended CBT and GET . (A recommendation which the PACE trial has helped lead too).
Then while I was appealing this disability pension knock back, I was forced to try to work part time for the dole or told I’d be cut off in which case I would of had no money at all to survive. This resulted in me putting my and others lives at risk at having to unsafely drive a car to try to get to part time work, (I lived in a country area where buses were not well accessable).. which resulted in me falling asleep at the wheel at a give way sign and slamming into another car.
That accident would not have occurred if I had got put onto disability pension and not been knocked back due to the not having done GET and CBT.
This PACE trial DECEPTION, has affect thousands of ME/CFS patients world wide and caused so much trouble for people from people not being able to get any medical care after drs tried to force them to do GET and CBT, to people like me getting knock backs from being able to get a disability pension for not doing GET and CBT and their reprecussions steaming from this.
Needless to say so many have had their health ruined further by this.
LikeLiked by 1 person
The PACE researchers and their supporting institutions have committed a massive fraud that has directly harmed many patients. I am confident that proper examination of the raw data will reveal the extent to which patients were harmed by their “treatments”, and this is why they have spent 250,000 pounds to hide the data.
It will take a class-action lawsuit to correct the injustice. I very much look forward to watching White and pals squirm under the heat of a vigorous cross examination.
LikeLiked by 1 person
Reblogged this on andrewpaulkleinblog and commented:
False and misleading reports about PACE Trial participants
LikeLiked by 1 person